Letter to AG of Georgia highlighting details of the hidden vaccinated death from Georgia in the Pfizer Covid 19 Vaccine trial
Dr. Jeyanthi Kunadhasan
Anaesthetist and Perioperative Physician
The Honourable Christopher M. Carr
Attorney-General of Georgia
40 Capitol Square, SW
Atlanta, GA 30334
USA
20th January 2025
RE: The Importance of Undisclosed Vaccinated Death from Georgia in the C4591001 Trial at the Vaccine and Related Biological Products Advisory Committee (VRBPAC) on December 10, 2020
Dear Attorney-General Carr,
My name is Dr. Jeyanthi Kunadhasan, and I am an anesthetist and perioperative physician in Australia. I draw your attention to the events below because I think this information is highly relevant to the people of Georgia, and I hope you will be able to carry it forward because it seems to be indicative of some very serious problems.
I am a member of a team of volunteers who investigated the original data from the Pfizer clinical trial CA4591001. This data was to be suppressed for 75 years until a Federal lawsuit forced its release on the Public Health and Medical Professionals for Transparency website[1]. This data formed the basis of the Food and Drug Administration’s Emergency Use Authorization (EUA) of Pfizer-BioNTech’s BNT162b2 mRNA COVID vaccine. Additionally, I am Treasurer of the Australian Medical Professionals Society.[2]
I coauthored “Forensic Analysis of the 38 Subject deaths in the 6-Month Interim Report of the Pfizer-BioNTech BNT162b2 mRNA Vaccine Clinical Trial,”[3] which is the first analysis of the Pfizer-BioNTech BNT162b2 mRNA vaccine original trial data by a group unaffiliated with the clinical trial sponsor. The information reported on here was revealed by our research presented in this journal article. Additionally, I authored Pfizer reports 42[4] and 76[5], available on Dailyclout.io.
I begin with an undisclosed trial-participant death from Georgia that occurred in the BNT162b2-vaccinated arm of Pfizer’s clinical trial as originally reported in War Room/Daily Clout Report 89, 'Researchers Find Pfizer Delayed Recording Vaccinated Deaths at Critical Juncture of EUA Process. Improper Delays in Reporting Deaths in the Vaccinated Led FDA to Misstate Vaccine’s Effectiveness, Influenced EUA Grant Decision.'[6]
As you are aware, the findings of the clinical trial C4591001, reportedly supporting the safety and efficacy of the BNT162b2 mRNA vaccine, have been published twice. Polack et al. first published findings on December 10, 2020, one day prior to the FDA’s issuance of EUA, entitled, “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine”[7]. Then, on September 15, 2021, Stephen J. Thomas, MD, et al. published, “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months.”[8] The Polack NEJM publication stated, "All the trial data were available to all the authors, who vouch for its accuracy and completeness and for adherence of the trial to the protocol, which is available with the full text of this article at NEJM.org. An independent data and safety monitoring board reviewed efficacy and unblinded safety data.” (Polack et al., 2020)
The Polack paper disclosed six deaths— two in the BNT162b2 Arm, and four in the Placebo Arm. In the journal article and the EUA approval documentation,[9] the six deaths covered the period of July 27, 2020, till November 14, 2020. This letter will demonstrate that Pfizer-BioNTech possessed records showing that eight deaths, four in the BNT162b2 Arm and four in the Placebo Arm, should have been disclosed by Pfizer to the FDA. In addition, the two undisclosed deaths presented a cardiac event signal in the clinical trials BNT162b2 recipients. One of the undisclosed deaths in the vaccinated arm of the trial occurred in Georgia.
Pfizer’s clinical trial protocol required that serious adverse events (SAE) be reported immediately upon awareness, and under no circumstances to exceed 24 hours, to Pfizer Safety on the Vaccine SAE Reporting Form.[10]The protocol required site investigators to record into the patient’s Case Report Form (CRF) all directly observed, and all spontaneously reported, adverse events and serious adverse events reported by the participant. In the case of a death, the next of kin/emergency contact would have been relied upon to inform a clinical trial site of a participant’s death, unlike the self-reporting of other adverse events.Prompt notification to the trial sponsor, by the clinical trial site investigator, of an SAE was essential to meet legal obligations and ethical responsibilities regarding the safety of participants and the safety of a study intervention under clinical investigation. The sponsor, in this case BioNTech, had a legal responsibility to notify both the local regulatory authority and other regulatory agencies about the safety of the study intervention under clinical investigation. The sponsor had to comply with country-specific regulatory requirements relating to safety reporting to the regulatory authority, Independent Review Boards (IRBs)/Ethics Committees (ECs), and investigators.
When we look at the table below, which is adapted from the “Forensic Analysis of the 38 Subject deaths in the 6-Month Interim Report of the Pfizer /BioNTech BNT162b2 mRNA Vaccine Clinical Trial”) (Michels et al., 2023), we can see, up to the data cutoff date of November 14, 2020, there were 11 deaths (six deaths in the vaccinated arm of the study and five in the placebo arm) in contrast to the 6 deaths publicly disclosed at the VRBPAC meeting and the Polack article. There was seemingly a capture rate of 33% (two reported deaths out of six deaths) in the vaccinated arm versus an 80% capture rate in the placebo arm (four reported deaths out of five deaths).
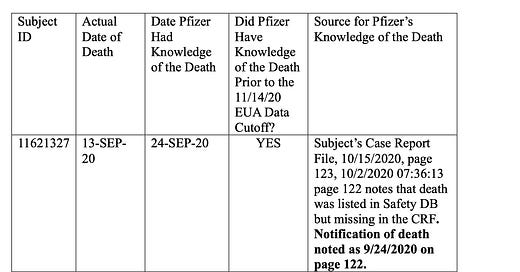
To determine how the discrepancies in reported deaths occurred, we started with the premise that, as of November 14, 2020, Pfizer-BioNTech was not informed of any deaths during the trial. The only way in which that could be validly disproved was demonstrating, through publicly available records, that Pfizer-BioNTech had knowledge of the deaths. By painstakingly going through each of the patient’s notes publicly available on the Public Health and Medical Professionals for Transparency (PHMPT) website, we identified the six dead subjects, whose deaths were reported in the initial Polack publication and VRBPAC meeting on December 10, 2020. They are vaccinated subjects 11621327 and 10071101, as well as the unvaccinated subjects 11521085, 12313972, 10661350, and 10811194. Their deaths occurred prior to November 14, 2020, and there was documentation of their deaths prior to November 14, 2020, in their respective CRFs.
Two BNT162b2 subjects whose deaths were included in the EUA submission:
Four placebo subjects whose deaths were included in the EUA submission:
Having established that Pfizer-BioNTech used the data entry of the notification of death in the CRF as the only data point that determined whether a death was reported, our investigation of the public records at that time could not provide answers as to why the other deaths were not reported.
However, the September 2023 Pfizer-BioNTech data released by the FDA provided a document named “125742_S1_M5_5351_c4591001-interim-mth6-narrative-sensitive.pdf”11 which included information showing that Pfizer-BioNTech was, in fact, informed of two additional deaths in the BNT162b2 arm of the trial well before the EUA data cutoff and that those deaths were not disclosed to the FDA. If the deaths had been disclosed at the time of the EUA, they would have shown that the BNT162b2 mRNA COVID vaccine intervention provided no reduction in deaths.
Subject 11201050, a 58-year-old woman, signed up for the Pfizer vaccine trial (C4591001) at the Meridian Clinical Research, 340 Eisenhower Drive, Suite 1200, Savannah 31406, Georgia. The Principal Investigator for this site is Dr Paul Bradley.
Her comorbidities included chronic back pain (since 2015), hypertension (since 2017), anxiety (since 2018), and type 2 diabetes mellitus (since 2018). Concomitant medications included metformin (since 2017) for type 2 diabetes mellitus; lisinopril (since 2017) and clonidine (since 2018) both for hypertension; and lorazepam (since 2018) for anxiety. 12
She received Dose 1 of BNT162b2 on 04 Aug 2020 and Dose 2 on 27 Aug 2020 (Day 24). On Nov 7th, 2020 (72 days after Dose 2), her husband called the clinical site and notified them that the subject had died in her sleep. The subject’s husband reported that the night before her death, she had taken an unspecified muscle relaxant and diazepam (Valium) for her chronic back pain; these medications were previously used by the subject. No symptoms or illnesses leading to the subject’s death were reported. The subject was not seen in the hospital. The coroner was called to pronounce death; an autopsy was not performed. This is a strange incuriosity into the cause of death of a 58-year-old woman who has died in her sleep, especially one enrolled in a clinical trial of a novel therapeutic.
According to the husband, the cause in the death certificate was stated as “cardiac arrest.” According to the investigator, there was no reasonable possibility it could be due to the study intervention. Pfizer concurred with the investigator’s causality assessment.
https://phmpt.org/wp-content/uploads/2023/09/125742_S1_M5_5351_c4591001-interim- mth6-narrative-sensitive.pdf page 75
Her husband called the clinical site itself on November 7th to inform them of her death. In the patient’s CRF, the date of collection/notification of death is Nov 7th, 2020. (see screenshot below).
.
h#ps://phmpt.org/wp-content/uploads/2023/05/125742_S1_M5_CRF_c4591001-1120- 11201050.pdf page 74
As such, this subject’s death should have been reported publicly in the VRBPAC meeting as well as the NEJM publication, as it had happened well within the reporting period prior to Nov 14th, 2020, and the clinical site documented it. Why wasn’t it?
Despite documenting receipt of her death on the day she died, the first entry elsewhere in her CRF regarding her death was on December 3rd, 2020.
h#ps://phmpt.org/wp-content/uploads/2023/05/125742_S1_M5_CRF_c4591001-1120- 11201050.pdf page 148-149.
As this was now after the data cutoff date, this was presumably the reason this death was not disclosed publicly. However, this is still prior to the December 10th VRBPAC meeting when the vaccine was approved. It seems as if the sponsor decided not to acknowledge this death, even though it occurred well within the reporting period concerned, with the clinical trial site acknowledging those facts. Other than obvious ethical issues raised, there are legal obligations to report deaths in a timely manner to local regulatory authorities to ensure safety of trial subjects.
Further inquiry is needed into –
the clinical trial investigator’s conclusion that this undisclosed death of a 58-year-old
woman in the vaccinated arm was not due to the vaccine. As the patient did not have any autopsy done, what was the evidentiary basis upon which the clinical investigator and the sponsor rely upon to form this conclusion?
The process in which the clinical site acknowledges notification of death on November 7, 2020, but this death is not disclosed publicly either in the VRBPAC meeting or the NEJM publication. Did the clinical site promptly, within 24 hours, fill in the Vaccine SAE Reporting Form and inform the clinical sponsors Pfizer and BioN Tech as they should have as per legal and ethical requirements?
Why this clinical site took 26 days, evident in their own documentation, to follow up on such a serious adverse event such as death? Has there been adequate regulatory oversight into this clinical site?
When did Pfizer and BioNTech acknowledge receipt of this death? When did Pfizer and BioNTech inform the regulatory authorities of this clinical trial subject’s death? Pfizer BioNTech submitted their application for the EUA to the FDA on 20 November 2020.
This Vaccine SAE Reporting Form is not currently publicly available, and compelling Pfizer/BioNTech and the clinical trial sites to provide all available information is the only way to establish the facts and timeline.
Below is a simple schematic diagram highlighting the important dates for this trial subject.
I want to highlight another undisclosed death of a vaccinated subject in this trial that also happened in the reporting period under consideration for the EUA, which similarly was not publicly disclosed. Subject 11141050, from Alliance for Multispecialty Research LLC, Newton, Kansas died on October 19, 2020. Despite timely notification of death by the emergency contact, there was a 37-day delay to this death being officially recorded in that subject’s CRF. This subject did have an autopsy done, of which the cause of death was “sudden cardiac death”. There is evidence in this subject’s CRF that this autopsy result was available prior to the December 10, 2020, VRBPAC meeting where Pfizer’s Covid 19 vaccine first received the EUA.13
I further scrutinised the deaths and autopsies performed overall in the trial. The vaccinated arm had 21 deaths, and only three of them (subjects 11141050, 11271112, and 11351033) had autopsies done.14 One autopsy resulted in the diagnosis of sudden cardiac death (subject 11141050), and the other two reports are still not available. Ten of the 21 deaths in the vaccinated subjects occurred in those who were found dead or suffered sudden adult death.15 Of those 10, only two (subjects 11141050 and 11271112) had autopsies done, with only one result (subject 11141050 - sudden cardiac death). The result of the autopsy for vaccinated subject 11271112 is still pending. There were 17 deaths in the placebo group, and only four (subjects 11521085, 11561124, 11681083, and 12314987) had autopsies. Of these, two (subjects 11561124 and 11681083) listed a cause of death. The other two results are still not available.
The autopsies or rather lack of autopsies in this trial become more pertinent considering the recently published systematic review of autopsy findings in deaths after COVID-19 vaccination. It found 73.9% of deaths were independently adjudicated as directly due to the vaccination, of which the primary cause of death included sudden cardiac death in 35%. 16
During the December 10, 2020, VRBPAC meeting, one reason cited for vaccine approval was "the known and potential benefits of the vaccine outweigh the known and potential risks of the vaccine when used for active immunization to prevent COVID-19 caused by SARS-CoV- 2 in individuals 16 years of age and older" (Vaccines and Related Biological Products Advisory Committee, 2020). Notably, the omission of the deaths and autopsy report from the vaccinated arm of the study at this critical juncture of EUA issuance raises substantial concerns about the overall safety reporting of Pfizer’s clinical trial. Patients who volunteered for the clinical trial likely did so, at least in part, in service of humanity. The failure to disclose the patients' deaths, despite timely notification by loved ones, constitutes a betrayal of their altruism and trust and deserves further investigation. It also raises considerable doubt about the reporting methods overall. These methods have led to medico-financial expenditure never seen in the world, and possibly on incorrect premises entirely.
Sincerely
Dr Jeyanthi Kunadhasan
MD (UKM) MMed (AnaesUM) FANZCA MMED(Monash)
[1] “Pfizer 16+ Documents.” Public Health and Medical Professionals for Transparency, Food and Drug Administration, 17 Feb. 2021, phmpt.org/pfizer-16-plus-documents/.
[2] “Australian Medical Professionals’ Society: A Society for Australian Medical Professionals.” AMPS, AMPS, amps.redunion.com.au/. Accessed 31 Dec. 2023.
[3] Michels, Corinne, et al. “Forensic Analysis of the 38 Subject Deaths in the 6-Month Interim Report of the Pfizer/Biontech Bnt162b2 Mrna Vaccine Clinical Trial.” International Journal of Vaccine Theory, Practice, and Research, International Journal of Vaccine Theory, Practice, and Research, 17 Oct. 2023, ijvtpr.com/index.php/IJVTPR/article/view/86.
[4] Kunadhasan, Jeyanthi, et al. “Report 42: Pfizer’s EUA Granted Based on Fewer than 0.4% of Clinical Trial Participants. FDA Ignored Disqualifying Protocol Deviations to Grant Eua.” DailyClout, DailyClout, 26 Sept. 2022, dailyclout.io/report-41-the-170-clinical-trial-participants-who-changed-the-world-pfizer-ignored-protocol-deviations-to-obtain-emergency-use-authorization-for-its-covid-19-mrna-vaccine/.
[5] Kunadhasan, Jeyanthi, and Ed Clark. “Report 76: Pfizer Had Data to Announce Its COVID-19 Vaccine’s Alleged ‘Efficacy’ in October 2020. Why Did Pfizer Delay the Announcement until November 9, 2020, Six Days after the 2020 U.S. Presidential Election?” DailyClout, DailyClout, 10 July 2023, dailyclout.io/report-76-pfizer-had-necessary-data-to-announce-its-covid-19-vaccines-alleged-efficacy-in-october-2020-why-did-pfizer-delay/.
[6] https://dailyclout.io/fda-granted-pfizer-eua-based-on-misrepresented-data/
[7] Polack, Fernando, et al. “Safety and Efficacy of the BNT162B2 Mrna Covid-19 Vaccine.” New England Journal of Medicine, nejm.org, 10 Dec. 2020, www.nejm.org/doi/full/10.1056/NEJMoa2034577.
[8] Thomas, Stephen J., et al. “Safety and Efficacy of the BNT162B2 Mrna Covid-19 Vaccine through 6 Months.” New England Journal of Medicine, nejm.org, 15 Sept. 2021, www.nejm.org/doi/full/10.1056/NEJMoa2110345.
[9] Naik, Ramachandra, et al. “Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization Review Memorandum.” FDA, fda.gov, 20 Nov. 2020, www.fda.gov/media/144416/download, p. 19.
[10] Pfizer. “A PHASE 1/2/3, PLACEBO-CONTROLLED, RANDOMIZED, OBSERVER-BLIND, DOSE-FINDING STUDY TO EVALUATE THE SAFETY, TOLERABILITY, IMMUNOGENICITY, AND EFFICACY OF SARS-COV-2 RNA VACCINE CANDIDATES AGAINST COVID-19 IN HEALTHY INDIVIDUALS.” Public Health and Medical Professionals for Transparency, PHMPT.org, 1 Mar. 2022, phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-fa-interim-protocol.pdf., p. 74.
11) Pfizer. “125742_S1_M5_5351_c4591001-Interim-Mth6-Narra<ve-Sensi<ve.Pdf.” Public Health and Medical Professionals for Transparency, phmpt.org, 1 Sept. 2023, phmpt.org/wp- content/uploads/2023/09/125742_S1_M5_5351_c4591001-interim-mth6-narra<ve-sensi<ve.pdf.
12) hjps://phmpt.org/wp-content/uploads/2023/09/125742_S1_M5_5351_c4591001-interim-mth6-narra<ve- sensi<ve.pdf page 7513 hjps://dailyclout.io/pfizer-did-not-disclose-a-kansas-vaccinated-sudden-cardiac-death-from-its-covid-19- clinical-trial-dr-jeyanthi-kunadhasans-lejer-to-kansas-ajorney-general-kris-kobach/
14 hjps://dailyclout.io/wp-content/uploads/Follow-up-lejer-Professor-Anthony-Lawler_17_5_24.pdf
15 Michels, Corinne, et al. “Forensic Analysis of the 38 Subject Deaths in the 6-Month Interim Report of the Pfizer/Biontech Bnt162b2 Mrna Vaccine Clinical Trial.” Interna:onal Journal of Vaccine Theory, Prac:ce, and Research, Interna<onal Journal of Vaccine Theory, Prac<ce, and Research, 17 Oct. 2023, ijvtpr.com/index.php/IJVTPR/ar<cle/view/86.
16 Science, Public Health Policy and the Law. 2024. “A Systema<c review of autopsy findings in deaths aher COVID-19 Vaccina<on - Science, Public Health policy.” November 17,
2024. hjps://publichealthpolicyjournal.com/a-systema<c-review-of-autopsy-findings-in-deaths-aher-covid-19- vaccina<on/.










Bravo as always JK !
This is some excellent work and I will be watching closely the response. Response or not, we are going to learn something about the nature of the beast.